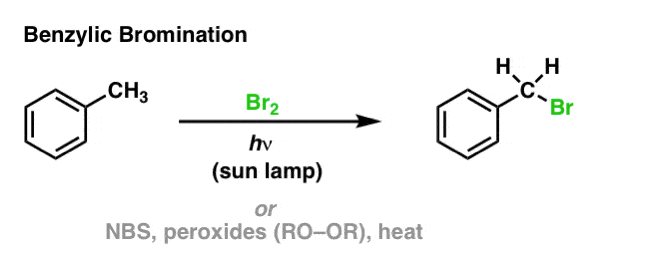
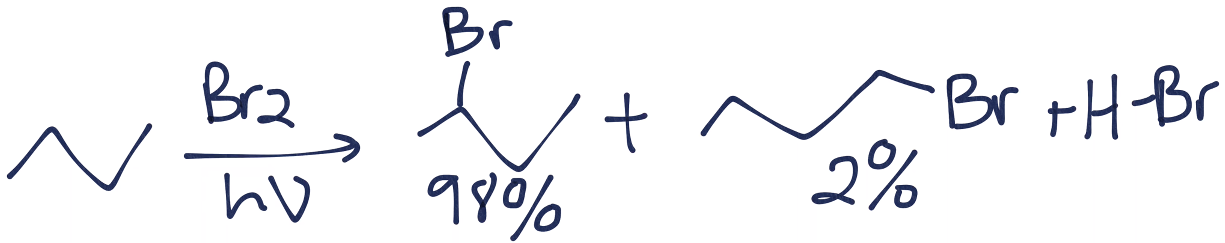When you react 2-methyl butane with Br_2 and UV light, the major product will be 2-Bromo-2-methyl butane. Is the statement true or false? | Homework.Study.com

When a chemist reacts 2-methylbutane with Br2 and UV light, the major product will be 2-bromo-3-methylbutane. a) true b) false | Homework.Study.com

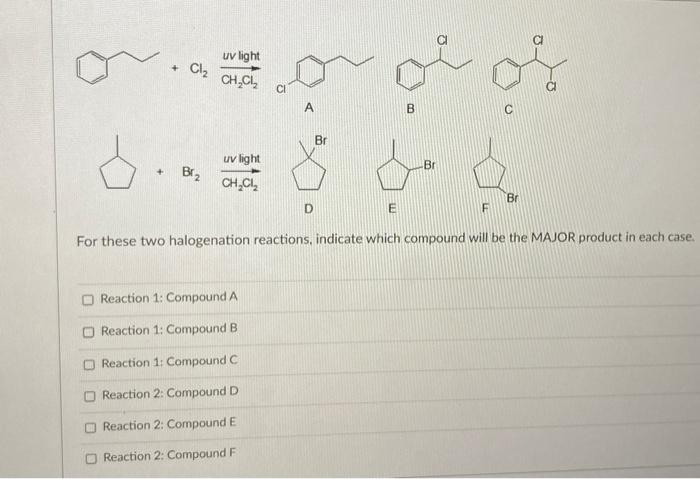
organic chemistry - Bromination of hexene in presence of UV light or heat - Chemistry Stack Exchange

Bromine water test: How can we differentiate between alkanes and alkenes experimentally (organic chemistry) — Understanding STEM

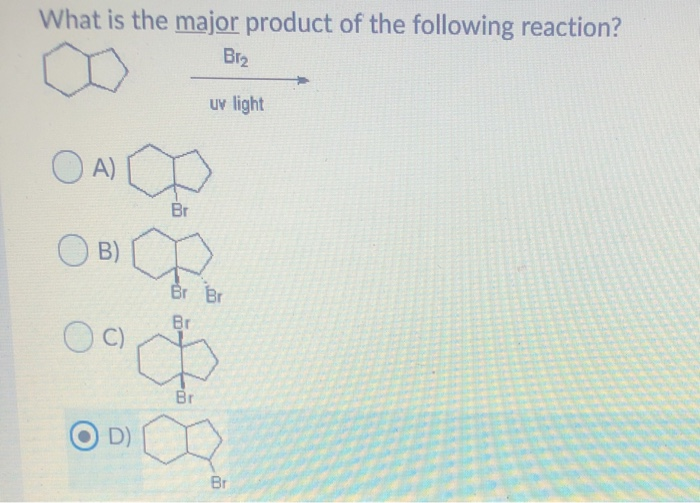
Bromination of cyclohexene under conditions given below yields : ltimg src=\"https://d10lpgp6xz60nq. - YouTube

Please show me mechanism with reason for the addition of Br2 in cyclohexene in the presence of heat and UV - Chemistry - Haloalkanes and Haloarenes - 9553865 | Meritnation.com
What is the mechanism for the reaction of cyclohexane with bromine in the presence of sunlight? - ECHEMI
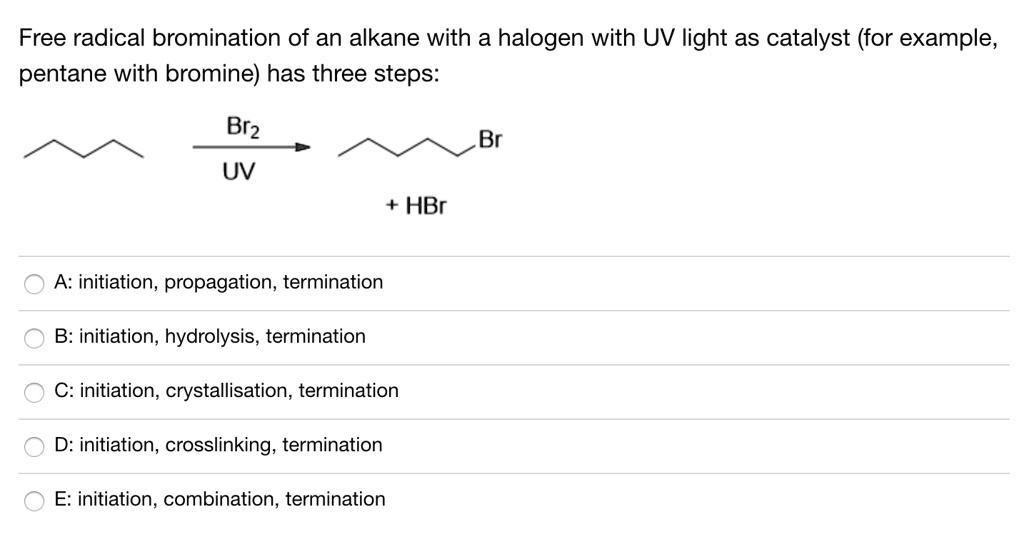

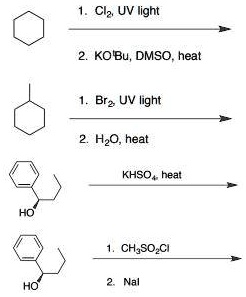
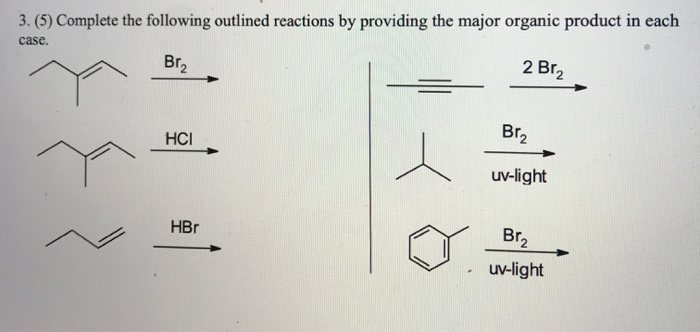

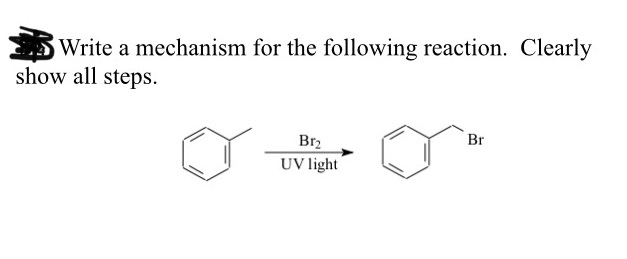
![CH3 - CH3 []Br2/hvA []LiAlH4B []Br2/hvC []Na/etherD The compound D is: CH3 - CH3 []Br2/hvA []LiAlH4B []Br2/hvC []Na/etherD The compound D is:](https://dwes9vv9u0550.cloudfront.net/images/3608069/07d40cff-181a-4ac8-bf10-20d0b19e64e0.jpg)