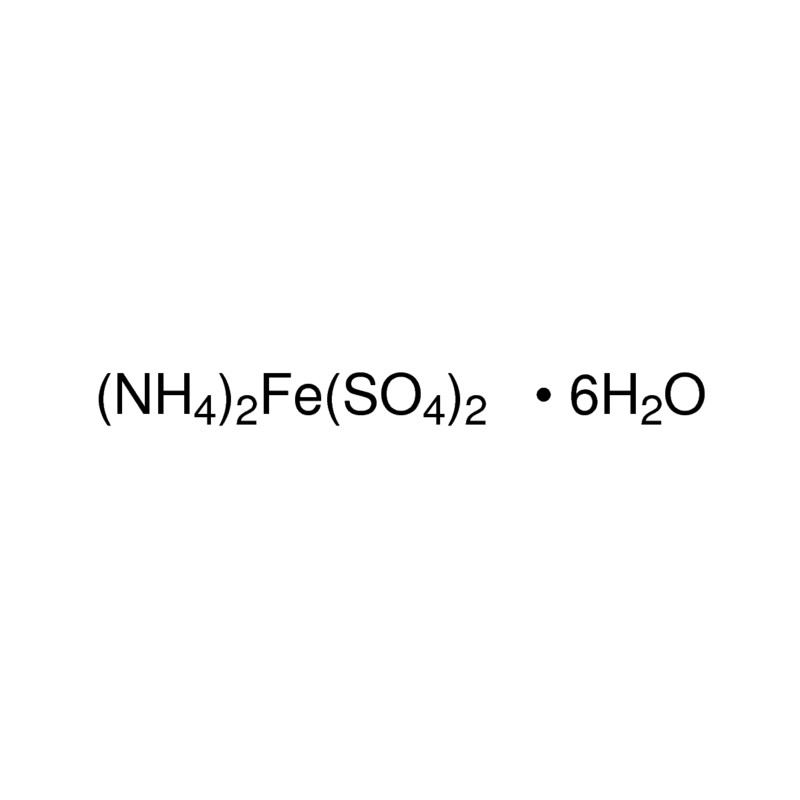The molecular formula of Mohr's salt is (NH.),SO.FeS0.6H,O(1) Find the number of atoms of each element.(2) - Brainly.in

The equivalent weight of Mohr's salt, F eSO 4· N H 42 SO 4· 6 H 2 O is equal to:A. Molar weight of Mohr's saltB. One fourth of the molar weightC.

The percentage composition of Mohr's salt is 14 32% Fe2+, 9 2% NH4+, 49% SO42- 27 57% H2O - Chemistry - Some Basic Concepts of Chemistry - 10320757 | Meritnation.com
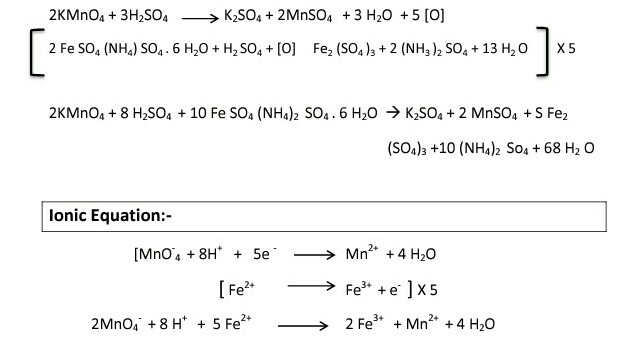
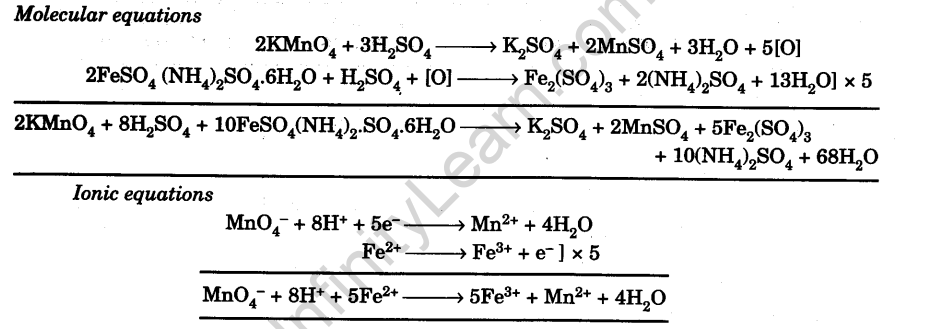
To determine the Molarity of KMnO4 solution by titrating it against a standard solution of Mohr's salt. (M/20 Mohr's salt solution).



![Solved] Mohr's salt is Solved] Mohr's salt is](https://d10lpgp6xz60nq.cloudfront.net/ss/web/2041461.jpg)